Welcome to a world of colour
- Designed to trap and kill pathogens on the surface of the curtain – reducing pathogen transfer.
- Effective against spores, bacteria and fungi.
- No laundry costs and 100% recyclable.
- Two attractive designs in multiple colour options – help create a welcoming environment for your patients.
Prevent
Cross-Contamination
Help Break the Chain of Infection
Layered Approach to Infection Control
Up to Two Year Lifespan
PRODUCT MENU
Product Details
Protecting your staff & patients all day, every day, Endurocide Antimicrobial Plus Hospital Printed Curtains feature patented biocidal technology. This helps reduce the spread of Healthcare Acquired Infections and break the chain of infection!
Features & benefits
- Kills top four pathogen groups found in hospitals*
- Breaks the chain of infection
- Light-weight, flexible & easy to use
- Significant cost savings
- Up to 50% fewer curtain changes required – saves time & reduces potential for injury
- Silver-additive free
- No laundry required
- Works with existing tracking
- Self-auditing & privacy labels
- 100% recyclable
Our technology is different...
Whilst traditional polyester curtains, natural fibre or short-life disposable curtains can easily become sources of pathogenic transmission within days of installation, our curtains are different..
Our technology in action...
Bacteria
are killed by both preventing their respiration and rupturing their cell membrane, then disrupting their cell metabolism and finally inhibiting the cell from reproducing
Fungi
are killed by both preventing their respiration and rupturing their cell membrane, then disrupting their cell metabolism and finally inhibiting reproductive functionality
Spores
are killed by piercing through the spore coats and cortex, so causing lethal DNA damage
Enveloped viruses
are inactivated by attacking and rupturing the virus's protein envelope, so stopping their ability to cause infections
See International Testing for details on pathogens tested.
Breaking the chain of infection
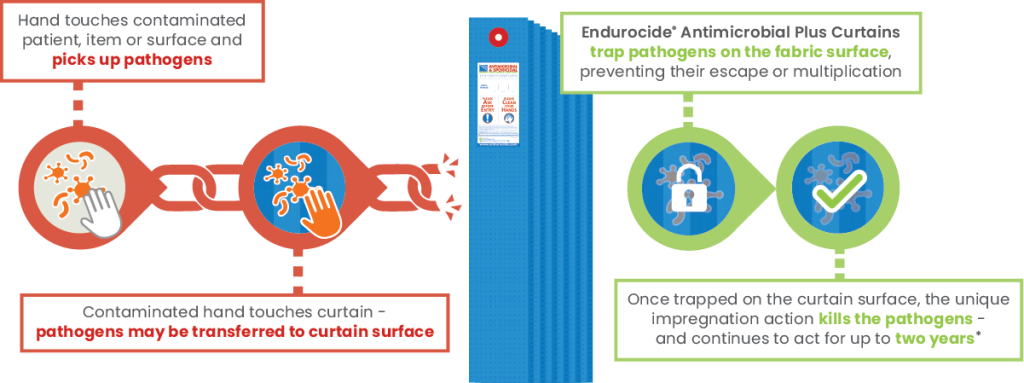
Endurocide® Antimicrobial Plus Printed Curtains prevent potential retransmission of pathogens to staff, patients, visitors, equipment, surfaces and any other items – breaking the chain of infection!
Behind the scenes
At the curtain manufacturing stage, the polypropylene curtain fabric is impregnated with our unique, patented Endurocide® Curtain Liquid.
This impregnation coats the curtain fabric creating a polymer layer which has a dual ‘static’ and ‘cidal’ action.
The ‘static’ action allows the curtain fabric to trap pathogens on the surface of the fabric and prevent them from multiplying, whilst the ‘cidal’ action then kills the pathogens – helping to break the chain of infection!
The dual mechanism of trapping and killing the top healthcare pathogen groups on the curtain surface is key – and what makes Endurocide® Antimicrobial Plus Curtains so unique
International Laboratory Testing
To validate our claims, Endurocide® Antimicrobial Plus Curtains – Printed Colours have been independently laboratory tested to international standards against pathogens including:
- C.difficile spores
- E.coli
- Salmonella typhimurium
- Vaccinia virus
- MRSA
- E.hirae
- Candida albicans
- Human Coronavirus
- VRE
- Klebsiella pneumoniae
- Measles virus
- H1N1 Swine Flu
Please note: our Endurocide® Antimicrobial Plus Curtains – Printed Colours use the same biocidal technology as our Antimicrobial Plus Curtains – Standard Colours to provide efficacy against spores, bacteria and fungi. However, due to the printing process, the test results differ between the products, as highlighted on this page.
Sporistatic & Bacteriostatic testing
Qualitative “Zone of Inhibition” tests demonstrate that the fabric is sporistatic and bacteriostatic and whether it can prevent pathogens from growing and reproducing on treated fabric. The International Standards for bacteriostatic & sporistatic fabrics are AATCC 147 and CG 147.
New curtains "box fresh" |
|||
Spores |
Standard |
||
|
CG 147 |
|
|
Bacteria |
Standard |
Standard |
|
|
CG 147 |
|
CG 147 |
|
CG 147 |
|
CG 147 |
|
CG 147 |
|
CG 147 |
Fungi |
Standard |
||
|
CG 147 |
|
|
Bactericidal testing
A quantitative test to demonstrate that the treated fabric is capable of killing pathogens.
The International Standard for biocidal treated fabrics is ISO 20743: 2013.
New curtains "box fresh" |
|||
Bacteria |
Standard |
Standard | |
|
ISO 20743 |
|
ISO 20743 |
Liquid treatment testing
The following testing has also been carried out on the liquid used to provide the antimicrobial and sporicidal coating on the curtains:
Enveloped viruses |
Standard |
Standard |
|
|
EN 14476 |
|
EN 14476 |
|
EN 14476 |
|
EN 14476 |
Fire Retardant Standards
Our curtains have been tested to the following International fire retardant standards:
Country/Region |
Standard |
|||||
|
BS 5867 Part 2 Types B & C: 2008 | |||||
|
CAN/ULC-S109; NFPA 701: 2010 | |||||
|
AS 2755.2: 1985; AS 1530.2-1993 Part 2 | |||||
Product Options
Endurocide® Antimicrobial Plus Printed Curtains are available in a number of different types and designs and with different hooks to suit your requirements.
Curtain types
Standard
For use with suspended ceiling rails.

Mesh Top
With NFPA 13 compliant mesh for sprinkler access with ceiling-fixed rails.

Mesh Cut†
An economical alternative to the Mesh Top curtain.

Long Drop
For use with ceiling-fixed rails.

† Note: Meshcut curtains do not meet US NFPA 13 specifications and are not suitable in areas that require mesh for sprinkler access.
Designs
These prints were designed exclusively for our Endurocide® Antimicrobial Plus Printed Curtains and we currently offer three different design prints in a variety of colour options.
'Simply Dotty' Design
The bright and vibrant dots in our Simply Dotty design were created to help bring some fun and colour into children’s wards, doctor’s surgeries, medical practices – or any ward. The varied sizes and irregular shapes in the design allow patients to focus in and out on the design, helping to calm the mind. Loved by all ages, this design brings a smile to patients, staff and visitors alike.

'Geometric' Design
This design offers patients and staff a subtle & calming pattern to focus on.
Antipodean Animals
You’ll see a selection of birds, animals and plants that can be found in New Zealand and Australia, including cute koalas, kangaroos, kiwi birds and even an echidna or two.
Although the new design features creatures from down under, we anticipate it will be popular in the Northern Hemisphere too. After all, who doesn’t love a kangaroo?
The new curtains are available on a blue or white background and work well with most hospital decors. Naturally, they offer the same performance as the other designs in the range.

Hanging Systems
Eyelet only
Quick-fit hooks
Large-top hooks
Wheeled hooks
Wide-wheeled hooks
Metal-bar wide-wheeled hooks
Eyelet extension hooks
Sizes
|
|
Standard |
Long drop |
Meshtop |
Meshcut |
|
2.0m | 2.6m | 2.55m | 2.55m |
|
7.5m |
7.5m |
7.5m |
7.5m |
|
5.55m |
5.55m |
5.55m |
5.55m |
|
3.75m |
3.75m |
3.75m |
3.75m |
|
N/A | N/A | 0.55m | 0.55m |
As a general rule, total curtain width needs to be 2 x length of curtain rail. Please note there is a +/-5% tolerance on all aspects of the curtains.
Order Code Selector
To generate an Order Code, please use our handy online Code Selector tool:
Where to Buy
Our products are available to purchase from a variety of sources, or please contact us today for a quote!
Resources
Videos
Please see below for links to videos featuring our curtains used internationally
Endurocide® Curtains help in the global fight against Coronavirus: Singapore
The video shows the new COVID-19 Treatment Facility constructed at Singapore Expo using our Endurocide® Curtains throughout
Endurocide® Curtains help in the global fight against Coronavirus: South Africa
The video shows the South African president Cyril Ramaphosa touring one of the many ICU wards established recently across South Africa which were using our Endurocide® Curtains
Brochures
use the links below to download our latest brochures
FAQs
At the curtain manufacturing stage, the polypropylene curtain fabric is impregnated with our unique, patented Endurocide® Curtain Liquid (which is a water-based formulation, based on a combination of active quaternary ammonium compounds) and a flame retardant chemical.
The Endurocide® Curtain Liquid coats the fabric with a biostatic and biocidal polymer layer. If pathogens come into contact with the fabric, the Endurocide® Curtain Liquid layer instantly starts working by trapping the pathogens on the fabric and preventing them from multiplying and being re-transmitted.
 |
|
Endurocide® Antimicrobial Plus Curtains are ready to work as soon as you take them out of the box and have been independently tested and shown to kill pathogens in seconds.
If you would like more information about the testing that has been conducted on our Endurocide® Antimicrobial Plus Printed Curtains, please visit our Our Technology or International Testing tabs, or contact us for more details on:
|
|
Studies have shown that up to 40% of HAIs can be attributed to the cross infection from the hands of health care personnel, who are contaminated either by direct contact with a patient or indirectly by touching contaminated surfaces1. It has also been shown that contaminated hands can transfer viruses to 5 more surfaces2 or 14 other subjects3.
On top of this, pathogens can survive on surfaces for long periods of time, with C.difficile spores and MRSA being shown to survive on surfaces for up to 5 months and 7 months respectively4. It is widely understood that hand hygiene protocols and surface disinfection procedures are essential in minimising the potential for this cross contamination.
However, what about the hospital curtain? What procedures are there to minimise its potential for contamination? If a health care worker touches a contaminated surface and picks up the contamination, then touches a hospital curtain, they have the potential to contaminate the hospital curtain. With some hospital curtains remaining in-situ for anywhere between one day to six months, these hospital curtains have the potential to become sources of transmission of infections for anyone who comes into contact with them.
Until now there was not much that you could do about this. However, Endurocide® Antimicrobial Plus Printed Hospital Curtains have changed this! By being tested and proven to kill spores, bacteria and fungi, Endurocide® Antimicrobial Plus Printed Hospital Curtains offer an effective barrier to pathogens and help hospitals maintain their hand and surface hygiene protocols – by minimising the potential for the hospital curtain to become a source of infection.
- Weber, David J; Rutala, William A; Miller, Melissa; Huslage, Kirk; Bennet-Sickbert, Emily “Role of hospital surfaces in the transmission of emerging health care-associated pathogens: Norovirus, Clostridium difficile, and Acinetobacter species” Am J Infect Control (2010) 38 S25-33
- Barker, J; Vipond, IB; Bloomfield, SF “Effects of cleaning and disinfection in reducing the spread of norovirus contamination via environmental surfaces” Journal of Hospital Infection (2004) 58:42-44
- Von Rheinbaben, F; Schunemann, S; Gross, T; Wolff, MH “Transmission of viruses via contact in a household setting: experiments using bacteriophage strain phiX!!74 as a model virus” Journal of Hospital Infection (2000) 46:61-66
- Kramer, Axel; Schwebke, Ingeborg and Kampf, Günter “How long do nosocomial pathogens persist on inanimate surfaces? A systematic review” BMC Infectious Diseases (2006), 6:130
Endurocide® Antimicrobial Plus Printed Curtains have been designed to be used wherever Infection Control & Infection Prevention is of paramount concern.
We would recommend that Endurocide® Antimicrobial Plus Printed Curtains should be used in, amongst others, Intensive Care Wards, High Dependency Units, Isolation Wards, Burn Units, Cardiology Wards, General Surgery Wards, Maternity Wards, Neonatal Wards and Orthopaedic Wards.
Please refer to the table below for more information:
Intensive care units |
| Gynaecology |
|
High dependency units |
| Obstetrics |
|
Infection disease units |
| Isolation wards |
|
Burns units |
| Accident and Emergency |
|
Cardiology wards |
| General wards |
|
Oncology wards |
| Day wards |
|
Operation suites |
| General practice wards |
|
General surgery wards |
| Occupational therapy |
|
Maternity & neonatal wards |
| Physiotherapy |
|
Renal units |
| Rheumatology |
|
All Endurocide® Curtains should be stored at ambient temperatures – i.e. at a maximum of 25°C max – and out of direct UV light.
If stored as above, Polypropylene remains stable for approximately 12 months, but after this time will start to gradually degrade. This degradation process will occur at a faster rate in the presence of warm/high temperatures, humid conditions and most importantly in the presence of UV light.
Any timescales provided/referenced are always offered as a guideline only and under no circumstances whatsoever constitute a guarantee.
Yes: every box of Endurocide® Curtains includes an Instruction Sheet in order to give our end users clear information on how to use and get the most out of their curtains.
- To download a copy, please click here.
Yes: a range of Endurocide® Antimicrobial Plus Printed Hospital Curtains are available from the NHS Supply Chain in England and Wales.
- For further information and links to the NHS Supply Chain website, please click here.
No: disposable curtains are designed to be replaced when visibly soiled and it is not advised to clean any of our curtains by any mechanical means.
Regarding Endurocide® Antimicrobial Plus Printed Curtains, wiping or cleaning them may affect the performance of the patented sporicidal formulation coated onto the curtain surface. The curtains are designed to kill pathogens in-situ and do not require any cleaning to improve their function. When any of our curtains become visibly soiled, they should always be replaced.
No: it is recommended that Endurocide® Antimicrobial Plus Printed Curtains are taken down when disinfecting with a misting/fogging machine.
The peroxide used in fogging/misting operations may react with the patented formulation coated onto the curtain’s surface and over time reduce the effectiveness against pathogens. The use of peroxide is also likely to affect the labels affixed to the curtains, resulting in a visible discoloration of the information on the curtain label.
It is advised that Endurocide® Antimicrobial Plus Printed Curtains are not placed in areas where they are exposed to prolonged direct sunlight.
Although the curtains will still be effective against pathogens, the polypropylene material the curtains are made from is not UV resistant and may suffer accelerated ageing as a result: this will lead to a loss in tensile strength and elasticity of the curtains which will become visible as “pilling”.
Pilling is caused by the fibres in the polypropylene becoming brittle with UV exposure, breaking down and coming loose from the main fibre chains. This effect is normally only observed after several months in direct sunlight.
Any timescales provided/referenced are always offered as a guideline only and under no circumstances whatsoever constitute a guarantee.
Each hospital and sometimes individual wards (depending on the ward type and purpose) will have their own specific protocols and assessments regarding the changing of curtains and these should be adhered to.
However, Endurocide® Antimicrobial Plus Printed Curtains have been independently tested and shown to offer antimicrobial & sporicidal properties for up to two years after manufacture. These test results have been demonstrated in both laboratory ‘box fresh’ test results, as well as independent peer review tests demonstrating real-life, in-situ testing by hospitals over a period of two years. For more information on these tests please click here, or contact us directly and our Technical Team will be happy to chat through any questions you may have.
Please note that Endurocide® Antimicrobial Plus Hospital Curtains are designed to be disposable and as such use polypropylene fabric. Should the polypropylene fabric become visibly dirty/soiled, we would recommend that the curtains should be changed, as they are not designed to be cleaned or washed.
Any timescales provided/referenced are always offered as a guideline only and under no circumstances constitutes a guarantee. The length of curtain hanging time will depend on individual ward use.
All Endurocide® Curtains are manufactured from 100% recyclable polypropylene and are completely recyclable. However, as the curtains are designed to come in contact with potential hospital pathogens, we would recommend that after use Endurocide® Curtains be disposed of as hospital waste or in accordance with local regulations.
For those customers concerned with the whole life of their products, there is the possibility of incinerating the curtains after use for their calorific value. Polypropylene has a high calorific value and one full width curtain could potentially generate enough energy to light a standard light bulb for an hour and a half.
However, any hospital specific protocols or local regulations concerning the safe disposal of clinical waste should always be adhered to.
Yes: we have conducted an in-depth analysis of the individual chemical components present in our patented formulation which is then applied to our Endurocide® Antimicrobial Plus Printed Curtains as a chemical blend during the treatment process.
To assess the risk of these individual chemical components, we have undertaken a technical analysis of the data available in the public domain and based assessments upon potential absorption and body mass calculations.
For the purposes of our assessments:
- We calculated that each standard, full width curtain is 15m2 (width: 7.5m x height: 2m). The Endurocide® treatment blend is uniformly applied to the curtain and equally distributed across the surface.
- We chose an area of the curtain approximately twice the surface area of the average hand (0.05m2 or 500cm2) and then calculated the mg/m2 of chemical blend used in this area.
- We then assessed the mg/m2 quantity of chemical blend in this single contact area for:
- Acute Toxicity risks
- Repeated Dose risks
- Calculations were based on average weighted UK males and females.
- We then assessed the mg/m2 quantity of chemical blend in this single contact area for:
Conclusions:
| For acute exposure risk | We assessed both oral LD50 and dermal LD50 (chemical mg/kg body mass) and determined that there is not enough of any substance present in the single contact area to exceed the oral and dermal LD50 values. In all cases, the quantities of the components that we use in our formulation were significantly lower than the stated maximum LD50 limits. |
| For repeated dose toxicity risk | It was determined that none of the quantities of individual components used exceeded the “No observable Adverse Effect Level” limits. |
Glossary of Terms & Clarifications:
| Acute Toxicity | The adverse effects of a substance that results either from a single exposure or from multiple exposures in a short period of time (usually less than 24 hours.) |
| LD50 | Lethal dose (LD50) is the amount of an administered substance that kills 50 percent of a test group. It is typically expressed as milligrams of administered substance per kilogram of body mass. |
No: whilst the biocidal technology used on Endurocide® Antimicrobial Plus Printed Curtains has been independently tested and shown to have long lasting antimicrobial and sporicidal efficacy, the actual length of curtain use will depend on a variety of factors.
Such factors include, but are not limited to: individual hospital practices; the natural longevity of polypropylene; the risk of the curtain being soiled from items such as blood, urine and general spills; etc. Curtains should always be replaced when visibly soiled.
Any timescales provided/referenced are always offered as a guideline only and under no circumstances whatsoever constitute a guarantee.
Yes: all types of Endurocide® Hospital Curtains.
Our Endurocide® Curtains have been independently tested and meet the following International Fire Retardant Standards:
- UK & Europe – BS 5867 Part 2 Types B&C: 2008
- USA & Canada – NFPA 701: 2010 and CAN/ULC-S109
- Australia & New Zealand – AS 2755.2-1985 and AS 1530.2-1993 Part 2
For copies of the certificates, please contact us.
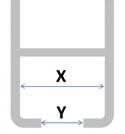
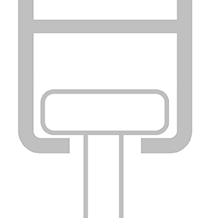
When choosing the type of hook you order with your curtain, it’s important that you order the correct size to fit your railing – and with so many types of railings available worldwide, this is sometimes easier said than done!
There are two ways to help ensure the hook will be suitable for your railing:
|  | ||
|  | ||
|  |
As a quick guide to our different types of hooks, please see the information below:
| Hook Type | Order Code | Image | Head width (A) | Neck width (B) | Suitable for railing system* |
| QF | | 10.0mm | 4.0mm | Silent Gliss 6100 |
| WH |  | 9.5mm | 4.5mm | Silent Gliss 6100 |
| LT | | 14.0mm | 3.0mm | Movatrack 100 |
| WWH | | 12.0mm | 4.5mm | Movatrack 100 |
| MWW |  | 12.0mm | 4.5mm | Movatrack 100 |
| U |  | N/A** | N/A** | Marlux Fast-Fit |
If you would like a sample pack of the different hooks we have available, please contact us.
Please note: it is important to ensure that the same hook is used throughout your Curtain. You should never mix hooks types or hook suppliers on one Curtain, as even similar type hooks can have significant differences in dimensions. To ensure uniform glide of your Curtain in the railing, rails should be regularly cleaned, free from obstacles or gaps and all hooks should be hung at the same height. Bio Technics accepts no responsibility for the performance of railings.
* The railings given are examples of commonly available systems – it is not meant to suggest that this is the only system that these hooks are suitable for use with.
** The X and Y measurements are not applicable to our U-Type hooks as these are designed to fit over the railing.
Endurocide® Antimicrobial Plus Printed Curtains are available supplied with eyelets only or with a choice of seven different hanging systems so will fit work with most popular track systems.
- For further information on the Hanging Systems available, please refer to the Product Options tab.
Endurocide® Antimicrobial Plus Printed Curtains are available in the following pack sizes:
Standard | Long drop | Meshtop | Meshcut | |
| Case of 6 | Case of 5 | Case of 5 | Case of 5 |
| Case of 8 | Case of 7 | Case of 7 | Case of 7 |
| Case of 12 | Case of 10 | Case of 10 | Case of 10 |
- For further details on the product options of our curtains, please see the Product Options tab.
All our Endurocide® Curtains are made from 100% polypropylene which has a very low environmental impact when compared with other types of synthetic or traditional materials.
In addition, the liquid used to impregnate our Endurocide® Antimicrobial & Sporicidal Curtains is water-based.
DISCLAIMER: Whilst Endurocide® Antimicrobial Plus Printed Curtains have been independently tested to be antimicrobially and sporicidally effective, the actual length of curtain use achieved will depend on a variety of factors including, but not limited to; individual hospital practices, the natural longevity of polypropylene and the risk of the curtain being soiled from items such as blood, urine and general spills etc. Curtains should always be replaced when visibly soiled.
Any timescales provided/referenced are always offered as a guideline only and under no circumstances whatsoever constitute a guarantee.

